White Matter Disorders (WMDs) may occur at all ages and have many different causes. Adult WMDs are more often acquired, either inflammatory in young adults or related to vascular risk factors in elderly. Children most often suffer from genetic WMDs, the leukodystrophies. The overall incidence of WMDs in children is ~1:1000, similar to that of Multiple Sclerosis (MS) in adults. WM damage leads to motor, cognitive and behavioral dysfunction. Although causes of WMDs are highly diverse, they share that the affected cells are glia (astrocytes and oligodendrocytes) and that myelin is either not formed or lost. For most WMDs no cure is available. Children with leukodystrophy die early, after a variable number of years. Better treatment is urgently needed for WMDs at all ages.
In collaboration with child neurologists Prof. dr. M.S. van der Knaap and Dr. N.I. Wolf of the Center for Children with WMDs at the VUMC we focus on two relative prevalent leukodystrophies: Vanishing White Matter (VWM) and 4H syndrome.
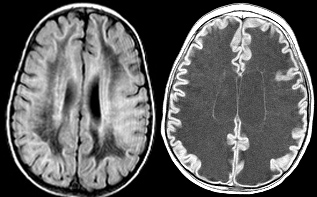
VWM is an autosomal recessive leukodystrophy. The main clinical features are cerebellar ataxia, spasticity and occasional seizures. MRI shows diffuse abnormality of the cerebral white matter with evidence of progressive rarefaction and cystic degeneration. VWM is caused by mutations in either one of the five genes (EIF2B1-5) encoding the five subunits of eukaryotic translation initiation factor eIF2B. The disease is progressive and leads to early death. There is no treatment for VWM. Cell therapy gives the prospect of halting progression or obtaining repair. This requires alternative cell sources allowing robust and safe cell replacement and correction of the cellular dysfunction. We explore the prospects for iPS cell-based therapy.
The 4H syndrome (hypomyelination, hypodontia, hypogonadotropic hypogonadism) is a recently described white matter disorder. The MRI of the brain shows profound lack of myelin deposited in the cerebral white matter (hypomyelination). It is the second most common hypomyelinating disorder. In their first decade, most patients are, in spite of the profound lack of myelin, only mildly affected with learning and motor problems. From the second decade, there is relentless decline. Patients die early. The disease is caused by mutations in one of 2 genes, POLR3A and POLR3B. How these lead to the 4H syndrome is not understood. We reprogram skin cells from 4H patients to nerve and myelin-producing cells to study the effect of the mutations on these cells. This is important for the development of a future therapy.
2026 © iPS Center. All Rights Reserved. Privacy Policy | Terms of Service

